New drug development demands significant time and financial investments, yet faces a staggering 90% failure rate. Among the critical factors influencing success, the physicochemical properties of drugs [1]—particularly polarity—play a pivotal role in determining permeability at target sites. Polar surface area (PSA), a measure of polarity, has long been used to predict pharmacokinetic properties and toxicity. Recently, exposed polar surface area (EPSA), an experimental method for quantifying PSA, has gained attention for its success in screening permeable cyclic peptides [2]. This article explores EPSA's role in optimizing passive permeability, its applications, and the principles of its detection via supercritical fluid chromatography (SFC).
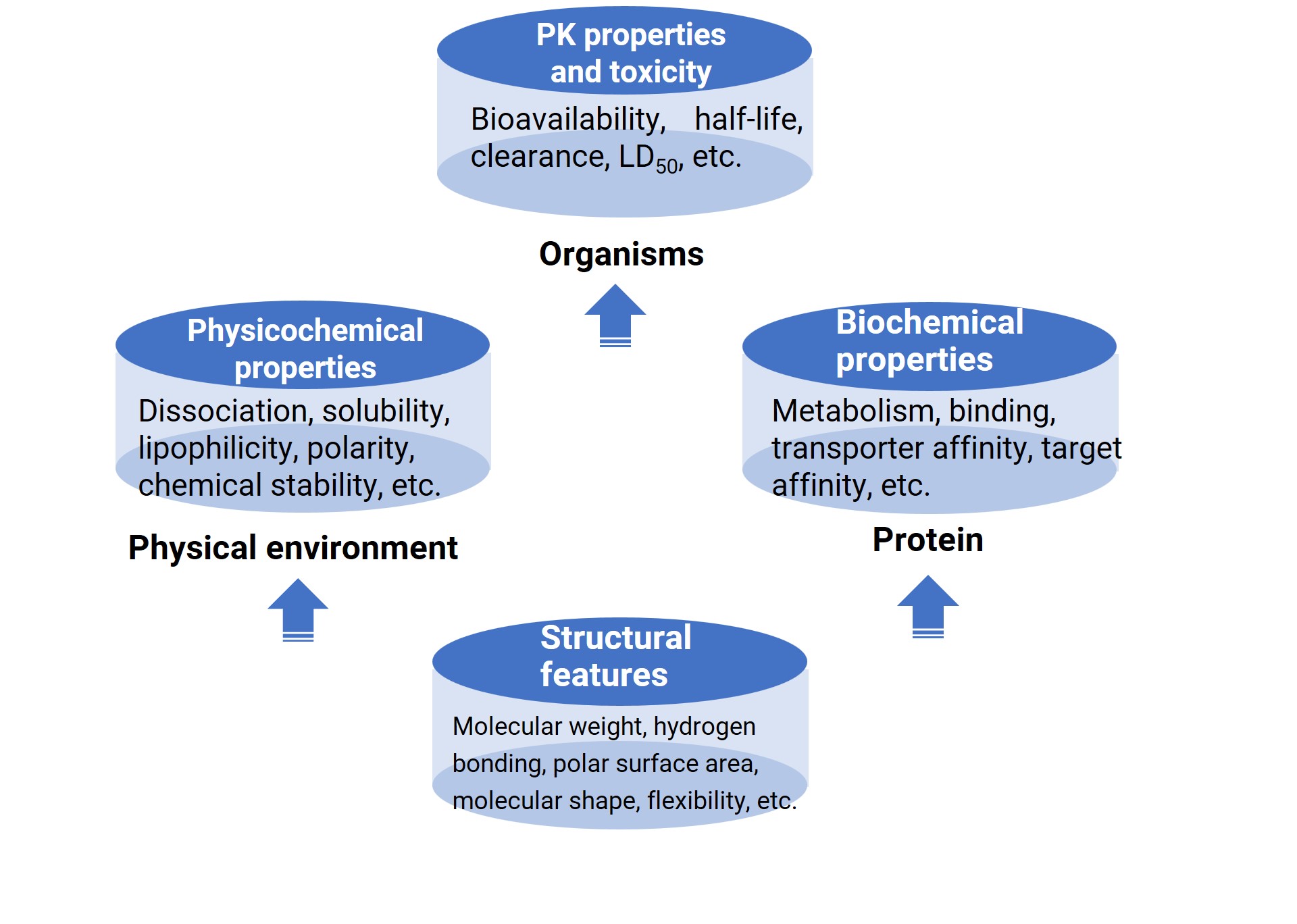
Figure 1. The relationship between compound structure, properties, pharmacokinetics, and toxicity.
What are PSA and EPSA?
PSA represents the sum of surface areas formed by polar atoms (e.g., oxygen, nitrogen) and their attached hydrogens (Figure 2). Since 1992, PSA has been instrumental in predicting absorption, distribution, metabolism, and excretion (ADME) properties [3,4]. Computational methods like dynamic PSA, static PSA, and topological PSA (TPSA) are commonly used, with TPSA favored for its speed and avoidance of complex 3D conformational analysis [5].
Unlike computational approaches, EPSA experimentally measures the "exposed" PSA, accounting for conformational changes and intramolecular hydrogen bonds (IMHBs) of molecules. It has proven especially valuable for large, flexible molecules such as cyclic peptides and proteolysis-targeting chimeras (PROTACs).
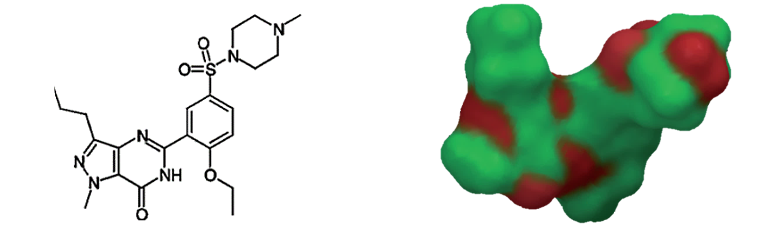
Figure 2. Sildenafil (left) and its van der Waals surface highlighting red polar regions (right) [5].
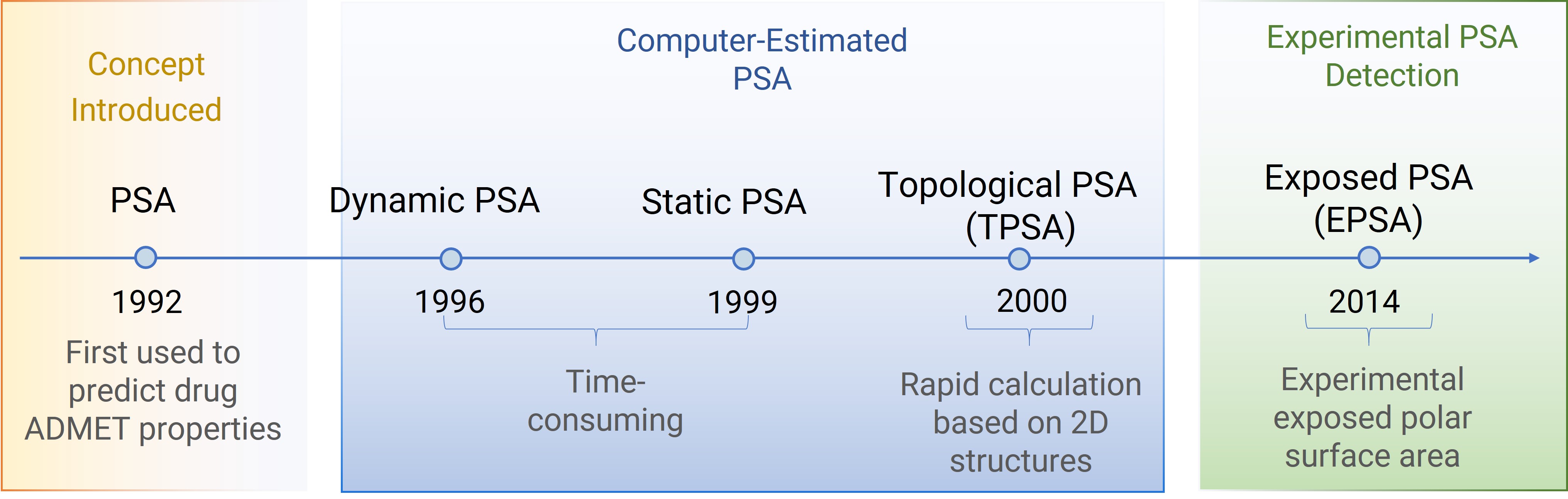
Figure 3. The development history of EPSA.
EPSA correlates chromatographic retention time with molecular polarity exposure based on SFC technology, enabling the detection of molecular polarity. SFC, a chromatographic analytical method developed rapidly in the 1980s, employs supercritical fluids as the mobile phase and solid adsorbents or polymers bonded to a carrier as the stationary phase. For certain pure substances, the critical point is reached when the gas and liquid phases coexist in equilibrium. When the temperature and pressure exceed the critical values, the substance exists as a supercritical fluid. EPSA, utilizing SFC technology, can be coupled with various detectors for quantitative and qualitative analysis. With higher diffusion coefficients and lower density of supercritical fluids as the mobile phase, SFC allows higher flow rates under lower column pressure. This results in higher column efficiency and shorter analysis times.
Applications of EPSA
EPSA was first validated in screening permeable cyclic peptides [2], a class of “beyond Rule of Five” (bRo5) molecules. Other oral bRo5 drugs include macrocycles, PROTACs, and natural products.
As FDA-approved bRo5 drugs rise (Figure 4) [6], their “molecular chameleon” behavior—adopting compact, low-PSA conformations in apolar environments—has emerged as a key permeability mechanism (Figure 5).

Figure 4. Analysis of the proportion of FDA-approved bRo5 and Ro5 drugs [6].
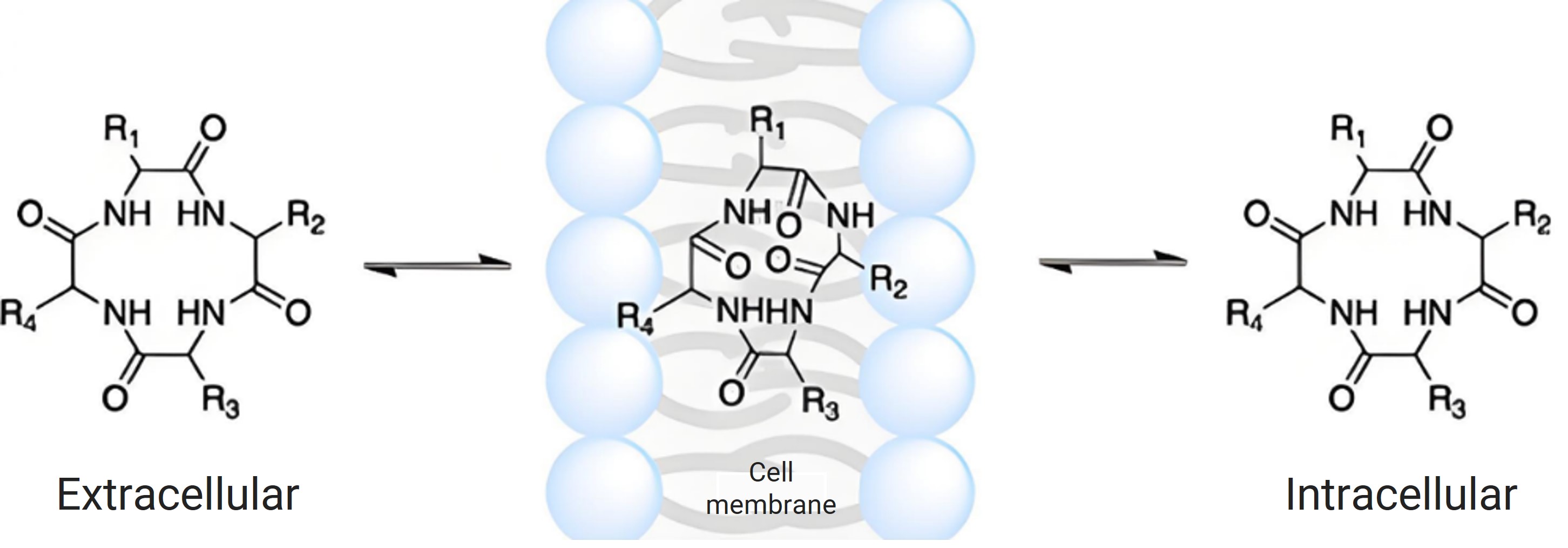
Figure 5. Mechanism of passive diffusion of chameleonic cyclic peptides across membranes [7].
This conformational flexibility, driven by IMHBs, allows bRo5 drugs to bind to large, flat, or grooved targets that are inaccessible to traditional Ro5 drugs (Figure 6) [7–9]. However, high molecular weight and polarity often hinder their permeability and oral bioavailability. EPSA addresses this challenge by accounting for conformational changes, providing a more accurate polarity assessment than 2D methods like TPSA. For example, cyclosporine A has a TPSA of 280 Ų but an EPSA of 72 Ų due to IMHBs masking its polar groups [10].

Figure 6. Binding site shapes for targets modulated by drugs in Ro5, bRo5, and eRo5 (extended rule of five) space [2].
EPSA in cyclic peptide screening
Researchers evaluated the permeability of 814 cyclic peptide drug molecules by combining EPSA with in vitro RRCK cell experiments. The results (Figure 7) showed that when the EPSA value is less than 80 Ų, the cyclic peptides exhibit moderate permeability; whereas when the EPSA value exceeds 100 Ų, they do not exhibit significant passive permeability. This study further indicated that EPSA could serve as a filter for discovering permeable cyclic peptides, with a cutoff value of 100 Ų [2].
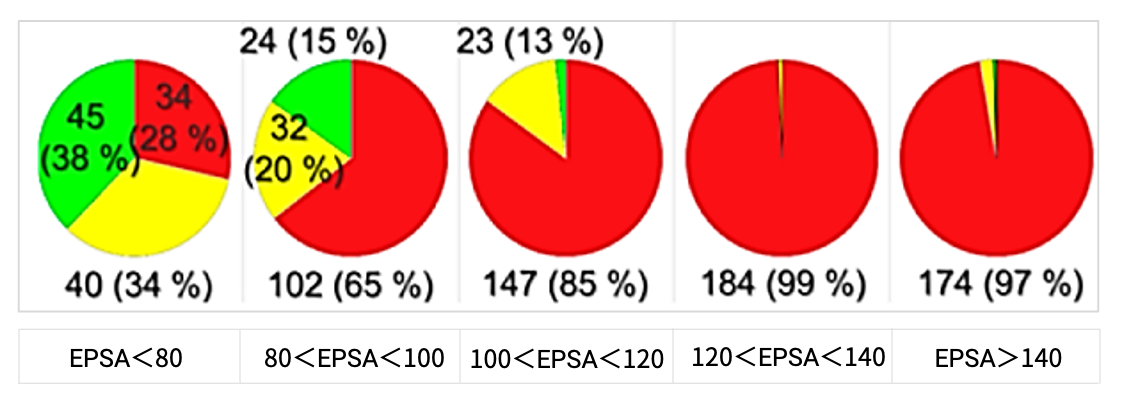
Figure 7. Pie chart analysis of EPSA and RRCK permeability data. (Papp < 1x10⁻⁶ cm/s, non-permeable (red); 1x10⁻⁶ cm/s < Papp < 5x10⁻⁶ cm/s, moderate permeability (yellow); Papp > 5x10⁻⁶ cm/s, permeable (green)).
EPSA in bRo5 oral PROTAC screening
Oral bioavailability is crucial for PROTAC molecules that do not conform to Lipinski’s “Rule of Five”. A study of 1,806 PROTACs at oral doses up to 30 mg/kg revealed that only 15% achieved oral bioavailabilities of ≥ 25% in rats. Analysis of four clinical PROTACs (ARV-766, ARV-110, KT-474, ARV-471) in mice, rats, and dogs, led to an empirical "oral PROTACs rule": eHDB ≤ 2, eHBA ≤ 16, ePSA ≤ 170, eRotB ≤ 13, MW ≤ 1000, chromLogD ≤ 7 [10].
Table 1. Experimental Physicochemical and Pharmacokinetic Properties of Four Clinical Oral PROTACs
|
calc./experiment |
ARV-766 |
ARV-110 |
KT-474 |
ARV-471 |
|
MW [Da] |
808 |
812 |
866 |
724 |
|
cLogP/LogD/chromLogD |
6.1/4.7/5.0 |
4.3/4.8/5.1 |
1.1/3.6/2.3 |
6.8/4.6/5.3 |
|
tPSA/ePSA [Å2] |
156/114 |
181/116 |
175/124 |
96/146 |
|
HBA/eHBA |
13/13 |
15/14 |
17/16 |
9/9 |
|
HBD/eHBD |
3/1 |
2/1 |
2/1 |
2/2 |
|
RotB/eRotB |
12/11 |
10/8 |
12/10 |
7/7 |
|
CL (m/r/d) [mL/min/kg] |
4.2/16/6.4 |
3.9/9.0/4.7 |
17/51/16 |
35/28/3.0 |
|
Vss (m/r/d) [L/kg] |
2.3/9.1/15 |
3.8/7.8/13 |
2.7/7.7/6.7 |
9.4/13/2.4 |
|
T1/2 (m/r/d) [h] |
6.8/7.4/31 |
13/9.3/32 |
4.0/2/6.8 |
6.2/15/11 |
|
F% (m/r/d) |
58/38/33 |
65/51/14 |
24/7/51 |
59/24/5 |
In fact, the application of EPSA is not limited to bRo5 drugs; it can also be used for screening Ro5 drugs. A retrospective study analyzed the screening process of 1,156 compounds [11], including both bRo5 and Ro5 drugs. The results indicated that EPSA, as a high-throughput filter in a screening funnel, could increase screening efficiency by 30% (Figure 8A) and improve the accuracy of permeability identification for bRo5 and Ro5 drugs by 20% and 40%, respectively (Figure 8B).
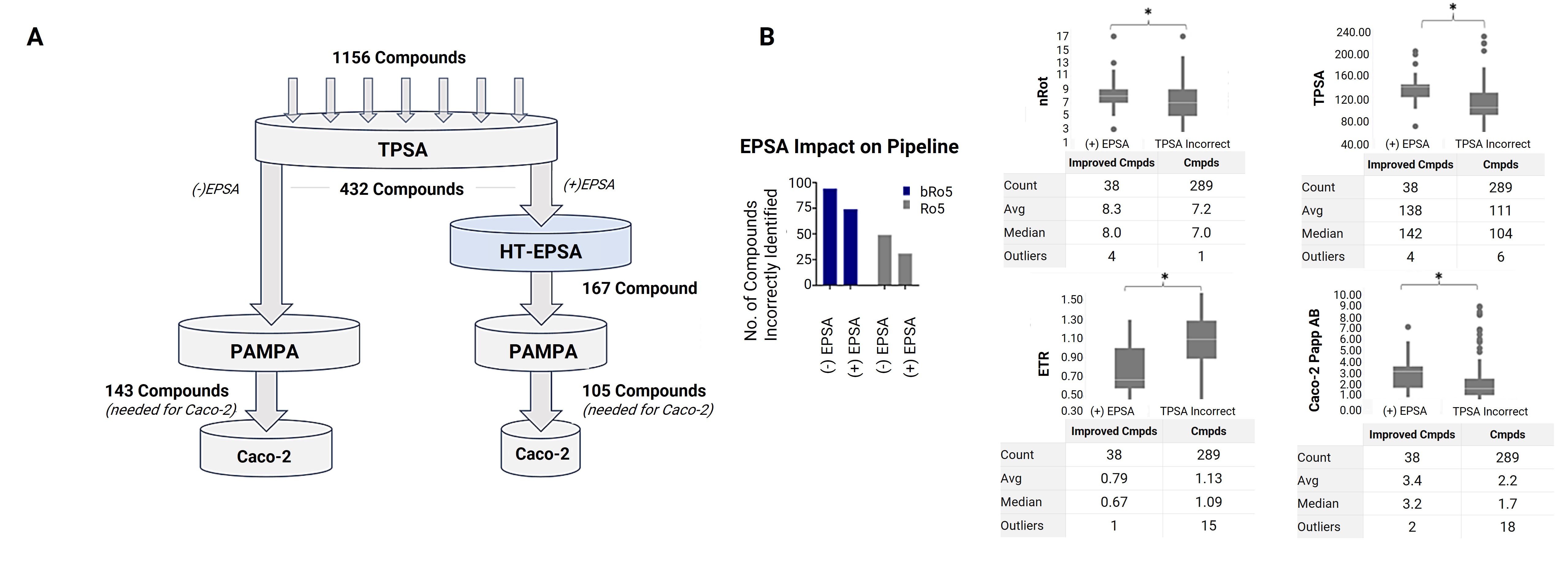
Figure 8. Retrospective funnel analysis of drug screening with (+) and without (-) high-throughput EPSA. (A) Funnel analysis of 1,156 compounds, with endpoints including subsequent assays of TPSA, EPSA, PAMPA, and Caco-2; EPSA corrected the compound screening priority. (B) Data analysis of EPSA’s impact on the drug screening funnel and improvement of the physicochemical properties incorrectly identified using TPSA. Asterisks (*) indicate statistical significance (p < 0.05).
Our practices in EPSA detection
Currently, the detection methods used for EPSA have matured, allowing for precise quantification of the polarity of chameleon molecules. During the detection process, attention must be paid to the following conditions:
#1 The detection environment must not disrupt IMHBs
The “molecular chameleon” property primarily relies on the intramolecular interactions that form IMHBs in apolar environments, which are associated with increased cellular permeability. Therefore, the potential for IMHB formation is an important consideration in drug design [12]. The detection mobile phase of EPSA uses supercritical carbon dioxide (scCO2), with the addition of the modifier methanol to create an unbiased environment with low dielectric constant solvents. This setup effectively simulates the hydrophobic structure within lipid bilayers, facilitating conformational changes in the analyte and the formation of IMHBs. The stationary phase is a silica-bonded chiral column featuring an (S)-valine moiety bound to (R)-1-(α-naphthyl)-ethylamine through a urea linker (Figure 9) [13]. The stationary phase contains numerous polar groups; the more interactions (primarily hydrogen bonds) that occur between the exposed polar groups of the analyte and the stationary phase, the longer the retention time (tR) and the greater the polarity.

Figure 9. Phenomenex Chirex 3014 stationary phase.
#2 Selection of ideal reference compounds
Existing studies using NMR and other techniques have confirmed that the reference compounds selected for EPSA experiments are prevented from forming IMHBs due to conformational restrictions [14]. Consequently, their EPSA values align with their TPSA, and a strong linear correlation exists between TPSA and retention time (tR). By substituting the tR of a test compound into the linear equation derived from the reference compounds’ TPSA-tR relationship, the EPSA value of the test compound can be calculated, covering a detection range of 61–230 Ų.
WuXi AppTec DMPK has successfully established an EPSA analytical platform using ultraperformance convergence chromatography (UPCC) coupled with mass spectrometry (MS) and ultraviolet (UV) detectors. Bland-Altman analysis verified the consistency between the EPSA values of 14 commercial compounds detected and those reported in the literature, indicating that this detection method is reliable (Figure 10). Using this method, we detected EPSA values for a range of bRo5 and Ro5 compounds, including cyclic peptides and PROTACs. By shortening the chromatography column and optimizing the elution gradient, we reduced the single-needle detection time to 6.5 minutes, tripling detection efficiency and improving linear fitting (Figure 11), thus achieving high-throughput and high-quality detection.

Figure 10. Consistency verification of EPSA detection values of 14 commercial compounds in WuXi AppTec DMPK laboratory against literature-reported EPSA values.

Figure 11. Comparison of linear fitting between high-throughput EPSA after method iteration and the original method.
The bottom line
EPSA addresses the limitations of traditional PSA calculations by incorporating conformational dynamics, providing a precise tool for designing chameleonic molecules with enhanced permeability. Its correlation with intestinal cell permeability surpasses that of TPSA, making it indispensable for oral bRo5 drug development. The refined EPSA platform developed by WuXi AppTec DMPK now supports rapid, high-quality screening of diverse molecules, accelerating drug discovery.
Authors: Ruxue Wang, Yongjing He, Li Qu, Dandan Liu, Quanli Feng, Cheng Tang
Talk to a WuXi AppTec expert today to get the support you need to achieve your drug development goals.
Committed to accelerating drug discovery and development, we offer a full range of discovery screening, preclinical development, clinical drug metabolism, and pharmacokinetic (DMPK) platforms and services. With research facilities in the United States (New Jersey) and China (Shanghai, Suzhou, Nanjing, and Nantong), 1,000+ scientists, and over fifteen years of experience in Investigational New Drug (IND) application, our DMPK team at WuXi AppTec are serving 1,600+ global clients, and have successfully supported 1,700+ IND applications.
Reference
[1] Sun D, Gao W, Hu H, et al. Why 90% of clinical drug development fails and how to improve it? [J]. Acta Pharm Sin B, 2022, 12(7):3049-3062.
[2] Goetz G H, Philippe L, Shapiro M J. EPSA: A Novel Supercritical Fluid Chromatography Technique Enabling the Design of Permeable Cyclic Peptides[J]. ACS Medicinal Chemistry Letters, 2014, 5(10):1167-1172.
[3] Papageorgiou C, Camenisch G, Borer X. Cell permeability as a parameter for lead generation in the protein Tyrosine kinase inhibition field[J]. Bio-organic & Medicinal Chemistry Letters, 2001, 11(12):1549-1552.
[4] Takle A K, Bamford M J, Davies S, et al. The identification of potent, selective, and CNS penetrant furan-based inhibitors of B-Raf kinase[J]. Bio-organic & Medicinal Chemistry Letters, 2008, 18(15):4373-4376.
[5] Clark, David E. What has polar surface area ever done for drug discovery? [J]. Future Medicinal Chemistry, 2011, 3(4):469-484.
[6] Stegemann S, Moreton C, Svanbäck S, et al. Trends in oral small-molecule drug discovery and product development based on product launches before and after the Rule of Five[J]. Drug Discovery Today, 2023, 28(2): 103344.
[7] Buckton L K, Rahimi M N, McAlpine S R. Cyclic peptides as drugs for intracellular targets: the next frontier in peptide therapeutic development[J]. Chemistry–A European Journal, 2021, 27(5): 1487-1513.
[8] Zheng, Jie, Doak, et al. How Beyond Rule of 5 Drugs and Clinical Candidates Bind to Their Targets[J]. Journal of Medicinal Chemistry, 2016, 59, 2312-2327.
[9] Villar E A, Beglov D, Chennamadhavuni S, et al. How proteins bind macrocycles[J]. Nature Chemical Biology, 2014, 10(9).
[10] Schade M, Scott J S, Hayhow T G, et al. Structural and Physicochemical Features of Oral PROTACs[J]. Journal of Medicinal Chemistry, 2024(15):67.
[11] Yue ting Wang, Edward Price, Mei Feng, et al. High-Throughput SFC-MS/MS Method to Measure EPSA and Predict Human Permeability[J]. Journal of Medicinal Chemistry, 2024, 67, 13765-13777.
[12] Caron G, Kihlberg J, Goetz G H, et al. Steering New Drug Discovery Campaigns: Permeability, Solubility, and Physicochemical Properties in the bRo5 Chemical Space[J]. ACS Medicinal Chemistry Letters, 2021, 12(1):13-23.
[13] Marziale A .6. SFC as a novel approach to assess polarity and identify intramolecular hydrogen bonding: Volume 1[M]. 2018.
[14] Goetz G H, Farrell W, Shalaeva M, et al. High throughput method for the indirect detection of intramolecular hydrogen bonding[J]. Journal of Medicinal Chemistry, 2014, 57(7):2920-2929.
Related Services and Platforms




-

 In Vitro ADME ServicesLearn More
In Vitro ADME ServicesLearn More -

 Novel Drug Modalities DMPK Enabling PlatformsLearn More
Novel Drug Modalities DMPK Enabling PlatformsLearn More -

 Physicochemical Property StudyLearn More
Physicochemical Property StudyLearn More -

 Permeability and Transporter StudyLearn More
Permeability and Transporter StudyLearn More -

 Drug Distribution and Protein Binding StudiesLearn More
Drug Distribution and Protein Binding StudiesLearn More -

 Metabolic Stability StudyLearn More
Metabolic Stability StudyLearn More -

 Drug Interactions StudyLearn More
Drug Interactions StudyLearn More
Stay Connected
Keep up with the latest news and insights.